COMPANY
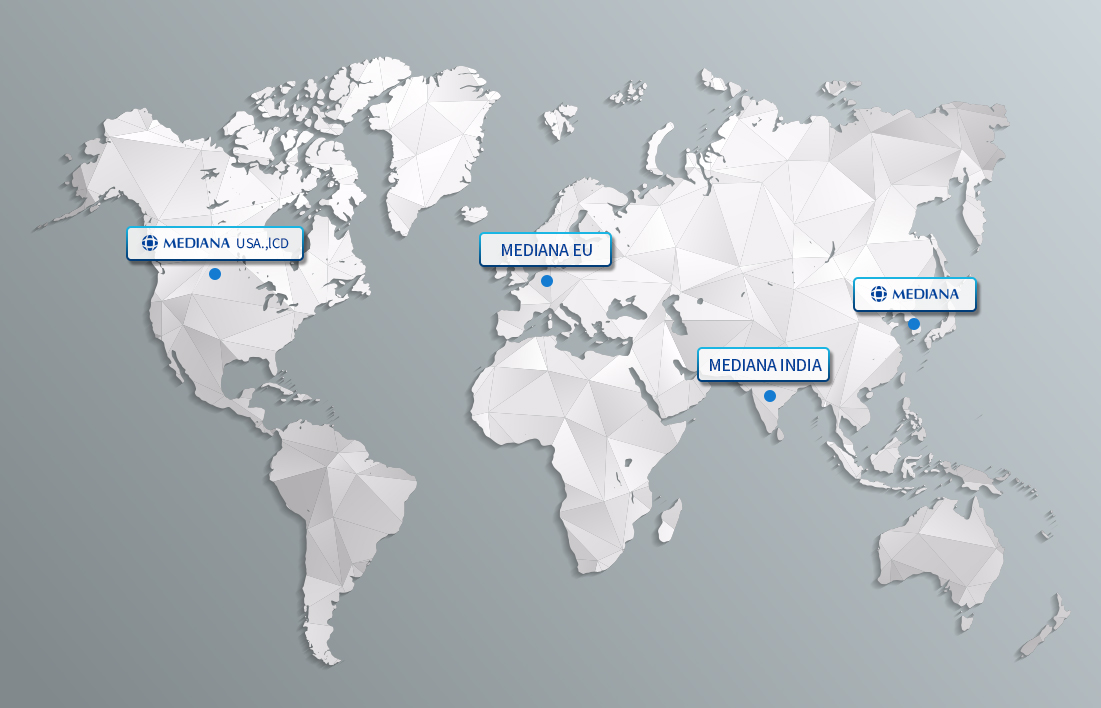
GLOBAL NETWORK
Mediana is a specialized manufacturer of
medical devices and is exporting ODM and
also our own branded products to all over the world.
Mediana’s agencies are located in over 80 countries around the world. Over 79% of our total revenue is generated from exports. In addition to Mediana, which serves as the head office of operations, subsidiaries are present in the USA, Germany, India and Brazil..
PARTNERS






CERTIFICATIONS
-
ISO13485:2016 / EN ISO13485:2016 / CE Mark
Obtain from BSI in May 2020 -
CE Mark
a quality management certificate that assures safety to European consumers and environmental protection -
MDSAP ISO13485:2016
Quality Management System certificate of MDSAP(Medical Device Single Audit Program) for Australia, Brazil, Canada, Japan and USA -
KGMP(Korea Good Manufacturing Practice)
Quality Management System certificate of Korea -
FDA : Food and Drug Administration
FDA 510(k) cleared GMP(Good Manufacturing Practice) compliance -
CB scheme certificate
CB Scheme, established by the International Electrotechnical Committee for Conformity Testing to Standards for Electrical Equipment (IECEE), provides a means for the mutual acceptance of test reports among participating safety certification organizations. -
UL certificate (NRTL)
UL certification derives from Underwriter’s Laboratory, an organization that tests and certifies electrical devices and appliances for consumer safety.UL is recognized by Nationally Recognized Testing Laboratory(NRTL), which determines that specific equipment and materials ("products") meet consensus-based standards of safety to provide the assurance, required by OSHA in U.S.




